

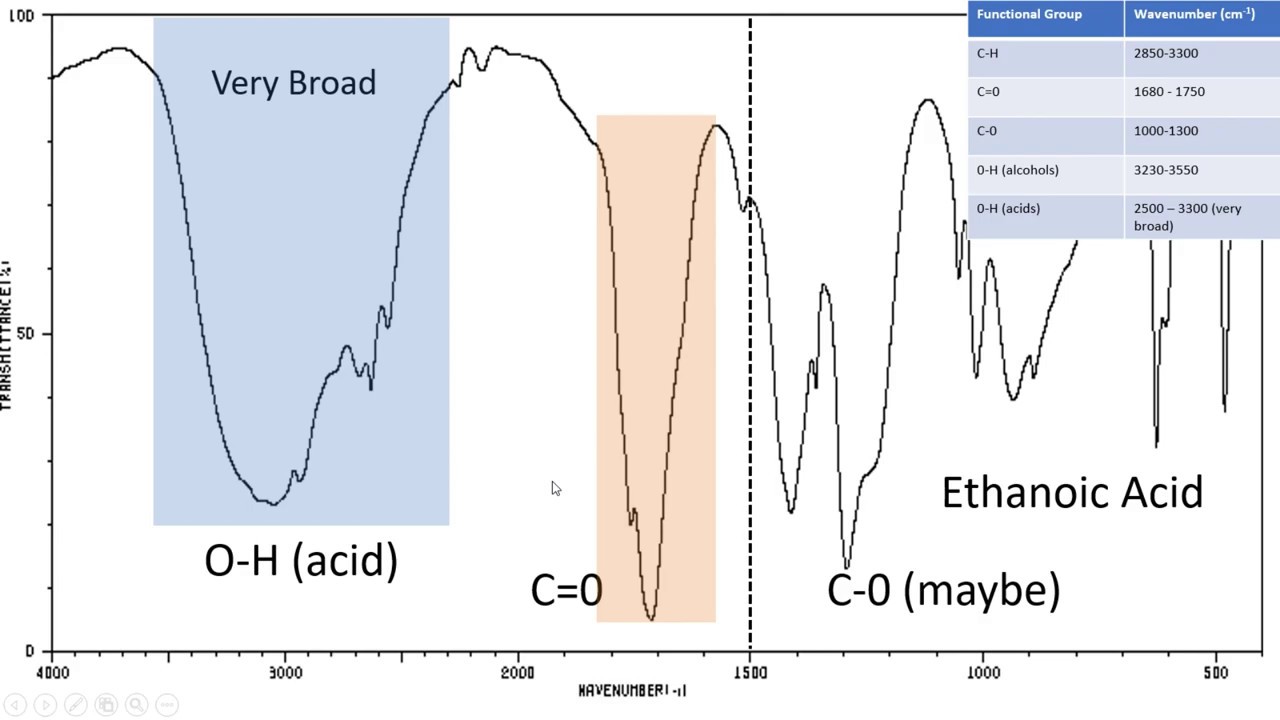
However, each type of bond will absorb IR radiation within a specific frequency range, and will have a common peak shape and absorption strength. The absorbed frequencies depend on the identity and electronic environment of the bonds, giving each molecule a characteristic spectrum. In this type of spectrum, the peaks are inverted, as they represent a decrease in transmitted light at that frequency. This is generally represented with a spectrum of percent IR radiation transmitted through the sample at a given frequency in wavenumbers. An IR spectrophotometer can measure which frequencies are absorbed. Molecules with certain types of covalent bonds can absorb IR radiation, causing the bonds to vibrate. Infrared, or IR, spectroscopy is a technique used to characterize covalent bonds. Characteristic IR frequencies of covalent bonds present in organic molecules.įigure 2. Diagram showing C - O double and C - C triple bond stretches and the resulting change in the dipole moment. This method enables one to directly examine solid or liquid analytes without further preparation.įigure 1. Typically, materials with a high refractive index are used, such as germanium and zinc selenide. In this experiment, the ATR sampling technique is used, where the infrared light reflects off the sample that is in contact with an ATR crystal multiple times. Notice the peak at 3,343 cm -1 for the N-H single bond and the peak at 1,695 cm -1 for the carbonyl groups. Figure 2 shows the IR spectrum of a Hantzsch ester. Table 1 shows some characteristic absorption frequencies. Hence, a carbonyl group stretch will show an intense band in the IR, and a symmetrical internal alkyne will show a small, if not invisible, band for stretching of the C-C triple bond ( Figure 1). The intensity of the infrared absorption is proportional to the change in the dipole moment when the bond stretches or compresses. On the other hand, a symmetrical alkyne does not have a net dipole moment because the two individual dipole moments on each side cancel each other. Hence, there is a net dipole moment resulting in a partial negative charge on oxygen and a partial positive charge on carbon. For example, in a C-O double bond (a carbonyl group), the electrons spend more time around the oxygen atom than the carbon atom because oxygen is more electronegative than carbon. If the electronegativities (the tendency to attract electrons) of the two atoms in a covalent bond are very different, a charge separation occurs that results in a dipole moment. When a molecule absorbs infrared light with a frequency that equals the natural vibrational frequency of a covalent bond, the energy from the radiation produces an increase in the amplitude of the bond vibration.
Infrared light is electromagnetic radiation with wavelengths ranging from 700 nm to 1 mm, which is consistent with the relative bond strengths. Double and triple bonds can be considered as stronger springs, so a C-O double bond has a higher stretching frequency than a C-O single bond. Thus, C-H, N-H, and O-H bonds have higher stretching frequencies than C-C and C-O bonds, as hydrogen is a light atom. The frequency is typically measured in wavenumbers, which are expressed in inverse centimeters (cm -1).įrom Equation 1, the frequency is proportional to the strength of the spring and inversely proportional to the masses of the objects. This frequency is given by Equation 1, where k is the force constant of the spring, c is the speed of light, and µ is the reduced mass ( Equation 2). Naturally, this bond stretches and compresses with a certain vibrational frequency. A covalent bond between two atoms can be thought of as two objects with masses m 1 and m 2 that are connected with a spring.


 0 kommentar(er)
0 kommentar(er)
